Total syntheses of Ganoderma-derived meroterpenoids, (−)-oregonensin A, (−)-chizhine E, (−)-applanatumol U, and (−)-ent-fornicin A†
Abstract
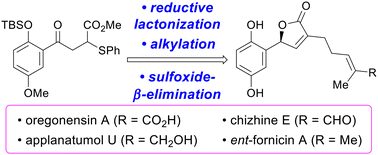
In this study, we report the total syntheses of Ganoderma-derived meroterpenoids, (−)-oregonensin A, (−)-chizhine E, (−)-applanatumol U, and (−)-ent-fornicin A. The 3-alkyl-5-aryl-γ-butenolide skeleton, a common motif of these meroterpenoids, was constructed through the enantioselective reductive lactonization of the γ-keto ester, alkylation, and sulfoxide-β-syn-elimination. This flexible approach enabled enantioselective access to these meroterpenoids with the longest linear sequence of 6–8 steps, and in 21–36% overall yield, respectively.


 Please wait while we load your content...
Please wait while we load your content...