Iron(iii)-catalyzed synthesis of indole–xanthydrol hybrid through oxidative cycloisomerization/hydroxylation reaction†
Abstract
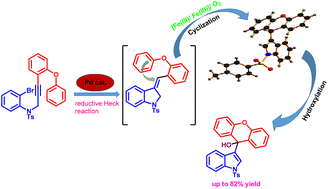
An efficient one-pot synthesis of an indole–xanthydrol hybrid is described in the presence of catalytic combinations of Fe(NO3)3/FeCl3. This strategy involves a series of reactions such as allylic oxidation, isomerisation, cyclisation and hydroxylation reactions in a tandem manner. This protocol offers several advantages including mild reaction conditions, operational simplicity, high selectivity, good yields and easily accessible starting materials. The synthetic utility of this protocol was further demonstrated by the one-pot synthesis of the highly substituted xanthene containing bis-indolylmethane derivative. The preliminary mechanistic studies reveal that the reaction is initiated by the generation of radicals in the presence of catalytic iron(III)-salts.


 Please wait while we load your content...
Please wait while we load your content...