A copper-catalyzed three-component reaction of dithioacetals with diazo ketones and ketimines†
Abstract
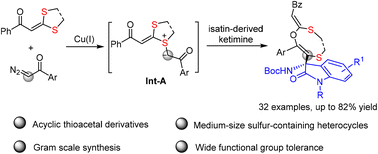
A copper-catalyzed three-component reaction of dithioacetals with diazo-ketones and ketimines has been reported. This reaction proceeds via trapping of the highly active sulfur ylide species, which are generated from thioacetal and carbene intermediates, with isatin-derived ketimine, providing an efficient protocol for the synthesis of acyclic thioacetal derivatives and medium-sized sulfur-containing heterocycles in good to high yields under mild reaction conditions with a broad substrate scope.


 Please wait while we load your content...
Please wait while we load your content...