Modification of proteins with azobenzene crosslinkers using reversible covalent bonds†
Abstract
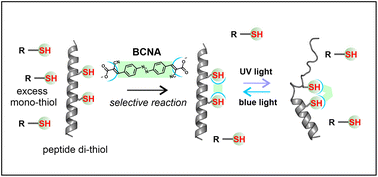
Thiol-reactive reagents designed for the chemical modification of proteins cannot, in general, be used directly for the modification of intracellular targets because the presence of millimolar concentrations of glutathione inside cells effectively outcompetes reaction with target thiols. Here we report an equilibrium, entropic strategy for achieving target selectivity using a cyanoacrylate-based thiol-reactive cross-linker (BCNA) with two reactive sites. This compound exhibits ≳200-fold selectivity for reaction with target peptides and proteins containing appropriately spaced pairs of thiols, versus reaction with mono-thiols. Photo-isomerization of the azobenzene moiety of the cross-linker can be used to affect the conformation of the target peptide or protein. This approach suggests a general strategy for the chemical modification of intracellular peptide and protein targets.


 Please wait while we load your content...
Please wait while we load your content...