Ambident reactivity of 5-aminopyrazoles towards donor–acceptor cyclopropanes†
Abstract
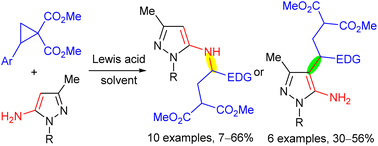
Lewis acid-catalysed reactions of donor–acceptor cyclopropanes with 1,3-disubstituted 5-aminopyrazoles were investigated. Under catalysis with gallium(III) chloride, products of the three-membered ring opening via a nucleophilic attack of the exocyclic amino group were obtained in a chemoselective manner. Oppositely, in the presence of scandium(III) triflate, products of either N-alkylation or C(4)-alkylation, or a mixture of both were formed. The products of the C(4) alkylation were transformed in one step into tetrahydropyrazolo[3,4-b]azepines that are attractive for medicinal chemistry and pharmacology.


 Please wait while we load your content...
Please wait while we load your content...