Transition metal-free reductive coupling of allylic sulfonylhydrazones with aryl boronic acids for C(sp3)–C(sp2) bond formation†
Abstract
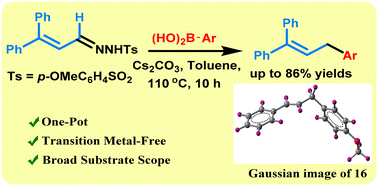
The reductive coupling between allylic sulfonylhydrazones and aryl boronic acids gives 1,3-diarylpropene systems with good to excellent yields. Simple reaction conditions, high yields, and good functional group tolerance are the salient features of this reaction which takes place without using any transition-metal catalysts and an inert atmosphere. The substituents on aryl boronic acid or allylic sulfonylhydrazone play a role in the isomerization of the double bond. The 3,3-diphenylacrylaldehyde derived allylic sulfonylhydrazone gives almost exclusively a single isomer.


 Please wait while we load your content...
Please wait while we load your content...