Synthesis and structure–activity relationship of berkeleylactone A-derived antibiotics†
Abstract
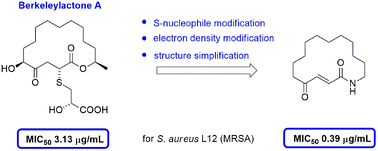
Berkeleylactone A is a potent 16-membered macrolactone antibiotic, recently isolated from a coculture of Berkeley Pit Lake fungi. Although its antimicrobial activity has already been investigated, little is known about the structure–activity relationship. Based on our previous synthetic studies, a series of berkeleylactone A derivatives were synthesized and evaluated for their in vitro antimicrobial activities against methicillin-sensitive and methicillin-resistant Staphylococcus aureus (MRSA) strains. Our data confirmed the essential role of the embedded conjugated system and suggest a reversible sulfa-protection of the Michael acceptor as a viable option. Structurally simplified achiral macrolactam 8 showed the best inhibitory activity against S. aureus L12 (MRSA) with MIC50 values of 0.39 μg mL−1, 8-fold lower than those of berkeleylactone A. These studies may be of value in the development of more advanced candidates for antibiotic applications.


 Please wait while we load your content...
Please wait while we load your content...