N-Heterocyclic carbene-catalysed homoenolate addition reaction to 3-cyano-2-imino-2H-chromenes: synthesis of C4-functionalized 2-amino-3-cyano-4H-chromene†
Abstract
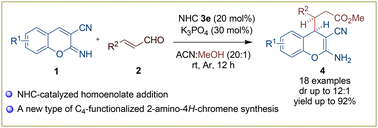
A diastereoselective N-heterocyclic carbene-catalysed reaction between enal and 3-cyano-2-imino-2H-chromene is described for accessing a new type of C4-functionalized 2-amino-3-cyano-4H-chromene. A wide variety of enals and iminochromenes afforded the desired homoenolate addition product 2-amino-4H-chromenes in good yields and with moderate to good diastereoselectivities.


 Please wait while we load your content...
Please wait while we load your content...