Impact of β-perfluoroalkyl substitution of proline on the proteolytic stability of its peptide derivatives†‡
Abstract
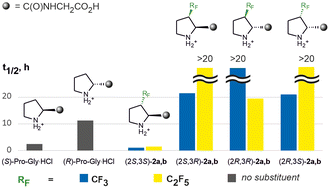
A series of all stereoisomers of β-CF3 or β-C2F5 substituted prolines and their dipeptide derivatives were synthesized. Mouse plasma stability assay was carried out to study the impact of fluoroalkyl substituents on the proteolytic stability of proline-derived peptides. The effect of the (R)-/(S)-configuration at the C-2 atom in combination with electronic and steric effects imposed by fluoroalkyl groups was addressed to rationalize the difference in the half-life stability of diastereomeric β-CF3–Pro–Gly and β-C2F5–Pro–Gly derivatives and compared to those of parent (S)-Pro–Gly and (R)-Pro–Gly dipeptides. The steric effect was predominant when the β-CF3 or β-C2F5 group was placed properly to create a spatial interference within the pockets of proteases, thereby protecting the substances from degradation (e.g., for cis-isomeric derivatives). Otherwise, a smaller electronic effect accelerating proteolysis was in charge (i.e., for the (2S,3S) isomers).


 Please wait while we load your content...
Please wait while we load your content...