Synthesis, computational investigation and biological evaluation of α,α-difluoromethyl ketones embodying pyrazole and isoxazole nuclei as COX inhibitors†
Abstract
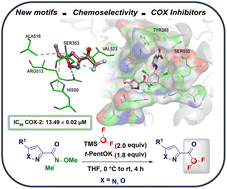
α,α-Difluoromethyl ketones (DFMKs) have emerged as currently investigated agents benefiting from the merging of chemico-physical features conferred by the constitutive elements (–CHF2 and carbonyl moietites). With a view to biological applications, the additional incorporation of heterocycles is a desirable property enabling the tuning of critical factors encompassing the pharmaco-dynamic and kinetic profiles. The underexplored assembling of α,α-difluoromethyl-heteroaromatic ketones is herein implemented via a conceptually intuitive Weinreb amide acylative transfer of a putative difluoromethyl-carbanion. To make the strategy productive, we adopted the commercially available TMSCHF2 pronucleophile – characterized by robust chemical stability and manipulability (bp 65 °C) – which upon Lewis-base mediated activation delivers the competent CHF2-nucleophile. The synthetic protocol was carried out on pyrazole- and isoxazole-based scaffolds, and a panel of heteroaryl-DFMKs was consequently developed as potential COX-inhibitors. In this sense, the bioisosterism deducted through docking studies between the widely expressed carboxylic group (in several clinically used COX inhibitors) and the –COCHF2 motif introduced herein supports this rationale. To confirm the docking results, all compounds were tested against both COX-1 and COX-2 enzyme isoforms showing activity in the micromolar range and a good selectivity index (SI). They were also evaluated for their biocompatibility using NIH/3T3 cells to which they did not show any significant toxicity.


 Please wait while we load your content...
Please wait while we load your content...