Efficient formation of hemoglobin bis-tetramers via selective acetylation of α-subunit amino groups by methyl acetyl phosphate†
Abstract
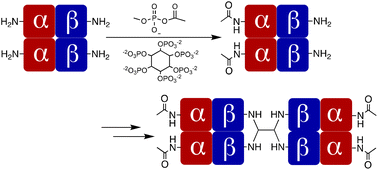
Chemical cross-linking of human adult hemoglobin (Hb) prevents dissociation of the tetrameric (αβ)2 protein into its constituent non-functional αβ dimers when present outside red cells, providing the possibility of being an acellular oxygen carrier in circulation. However, studies of cross-linked Hb (xlHb) in circulation established effects consistent with scavenging of endogenous nitric oxide, leading to hypertension. Bis-tetramers, composed of coupled Hb tetramers, are sufficiently large to avoid penetration of endothelia, thereby blocking access to endogenous nitric oxide. Cu(I)-catalyzed azide–alkyne cycloaddition (CuAAC) joins two azide-functionalized xlHbs to each end of a bis-alkyne to form bis-tetramers. The process critically depends on formation of a cross-link between lysyl amino groups of the β-subunits while avoiding reactions with amino groups in the α-subunits. Highly selective acetylation of α-subunit amino groups with methyl acetyl phosphate (MAP) effectively directs subsequent cross-linking to the β-subunits. This outcome leads to efficient production of hemoglobin bis-tetramers by CuAAC.


 Please wait while we load your content...
Please wait while we load your content...