pH-controlled regioselective nucleophilic ring-opening of epoxide: an improved process for the preparation of (R)-(−)- or (S)-(+)-3-hydroxytetrahydrofuran†
Abstract
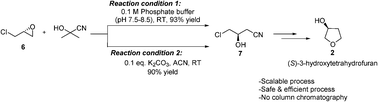
An environmentally benign, cost-effective and scalable process for the preparation of both the enantiomers of 3-hydroxytetrahydrofuran has been developed. pH-Controlled ring opening of enantiomerically pure epichlorohydrins with cyanohydrin is the key step of the process. The entire protocol does not require any column purification.


 Please wait while we load your content...
Please wait while we load your content...