Synthesis and biological evaluation of N-alkyl sulfonamides derived from polycyclic hydrocarbon scaffolds using a nitrogen-centered radical approach†
Abstract
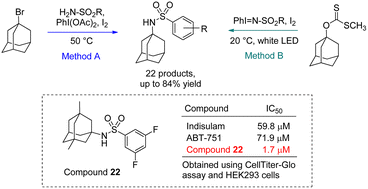
Polycyclic hydrocarbons (PH) provide intriguing potential as lipophilic scaffolds within medicinal chemistry, but are currently limited by the availability of synthetic tools for predictable modification of the PH unit. Herein we report the development of new methods for installation of a sulfonamide unit to PH cores. In the first method, a xanthate ester serves as reagent for aminosulfonation using pre-formed imidoiodinane as N-source. An investigation of the reaction mechanism was performed to implicate a process involving a N-centered radical. An additional method for sulfonamide installation is described that involves the use of commercially available reagents and operationally convenient conditions. Using the new synthetic methods, 22 compounds were prepared and screened for biological activity against 6 mammalian cell lines along with Gram-positive and Gram-negative bacterial strains. Results of the viability assays have identified compounds that exhibit higher potency than other known anticancer agents such as indisulam and ABT-751. Additionally, the physicochemical and drug-likeness properties of the synthesized compounds have been determined experimentally and using in silico predictive tools. The initial exploration into sulfonamide insertion into PH cores has resulted in a number of compounds that warrant further development to produce molecules with therapeutic value.


 Please wait while we load your content...
Please wait while we load your content...