Asymmetric synthesis of (+)-teratosphaerone B, its non-natural analogue and (+)-xylarenone using an ene- and naphthol reductase cascade†
Abstract
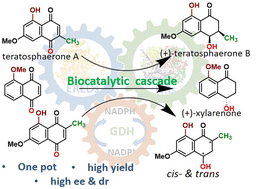
Herein, a one-pot bienzymatic cascade containing an ene and a naphthol reductase is developed. It is applied for the synthesis of (+)-(3R,4R)-teratosphaerone B, its non-natural regioisomer in both cis- and trans-forms and (+)-xylarenone by the reduction of chemically synthesized naphthoquinone precursors in high yields (76–92%) and excellent ee (>99%). This work implies similar biosynthetic steps in the formation of the synthesized natural products.


 Please wait while we load your content...
Please wait while we load your content...