CBr4 catalyzed activation of α,β-unsaturated ketones†
Abstract
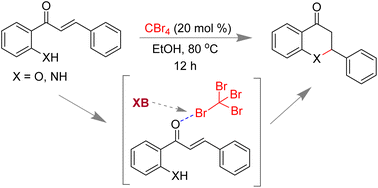
We have shown here that weak interactions such as halogen bonding (XB) can be used to activate the carbonyl group of α,β-unsaturated ketones. Carbon tetrabromide (CBr4) has been used as the sole reagent for the selective synthesis of flavanones and aza-flavanones from the corresponding 2′-hydroxy- and 2′-aminochalcones under metal-free and additive-free conditions. DFT calculations support the catalytic role of XB between the oxygen of chalcones and CBr4 in these reactions.


 Please wait while we load your content...
Please wait while we load your content...