Fluoride ion coordination-induced turn-on fluorescence of tailored N-methyl N-confused tripyrromonomethene analogues†
Abstract
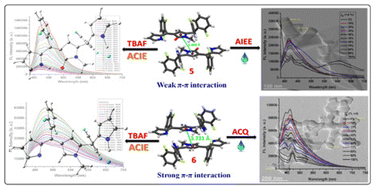
Structural isolation of two unprecedented AIEE/ACQ type fluorophores based on N-methyl N-confused tripyrromonomethene analogues exhibiting selective F− anion-coordination-induced-enhanced emission (ACIEE) with a detection limit of 10−7 M is reported. The intrinsic relation between molecular structures/molecular arrangements with (without) anion binding have been revealed at a deeper level via spectroscopic measurements and DFT level theoretical studies.


 Please wait while we load your content...
Please wait while we load your content...