Synthesis of fluoroolefin derivatives by nickel(ii)-catalyzed trifluorovinylation and 1,2,2-trifluoroethylation of cinnamyl alcohols†
Abstract
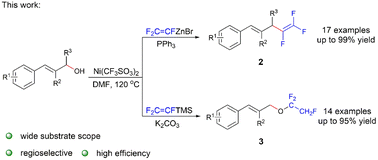
Nickel(II)-catalyzed regioselective trifluorovinylation and 1,2,2-trifluoroethylation of allyl alcohols with trifluorovinyl reagents were performed. The reaction of (E)-3-phenylprop-2-en-1-ol and trifluorovinylzinc bromide (TFVZ) afforded 1,2,2-trifluorovinyl diene in moderate to high yields. The reaction of (E)-3-phenylprop-2-en-1-ol and trifluorovinyl trimethylsilane (TFVTMS) resulted in the novel nucleophilic addition product 1,1,2-trifluoroethoxy aryl olefin.


 Please wait while we load your content...
Please wait while we load your content...