Solvent dependent hindered rotation versus epimerization in axially chiral thiohydantoin derivatives: an experimental and a computational study†
Abstract
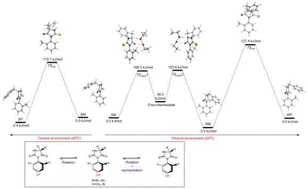
5-Benzyl-3-(o-aryl)-2-thiohydantoin and 5-isobutyl-3-(o-aryl)-2-thiohydantoin derivatives (o-aryl = o-tolyl and o-bromophenyl) have been synthesized by reacting o-aryl isothiocyanates with S-phenylalanine methyl ester hydrochloride or with S-leucine methyl ester hydrochloride in the presence of triethylamine (TEA). The synthesized compounds have a chirality center at C5 of the heterocyclic ring and a chirality axis, the N3–C(aryl) bond. The axially chiral compounds were shown to exist in unequal amounts of SM, SP, RM and RP stereoisomeric forms with a high prevalence of the P isomers over the M isomers. The isomeric assignments were done by comparing the 1H NMR spectra with the HPLC chromatograms. The stereoisomers were resolved micropreparatively by HPLC on chiral stationary phases and the interconversion of the single isomers has been investigated. The conversion type has been determined as epimerization or rotation by the HPLC analyses. It has been found that although the stereoisomers converted to each other only by rotation in toluene, in ethanol epimerization (racemization at C5 of the heteroring) was accompanied with rotation depending on the duration, temperature of the thermal interconversion experiment and the nature of the ortho substituent. The occurrence of epimerization was also proved through H/D exchange reactions via1H NMR experiments done in CD3OD. The rotation and epimerization mechanisms of synthesized compounds were further elucidated by Density Functional Theory (DFT) calculations at M062X/6-311 + G** level of theory and the results were shown to be in harmony with experimental findings.


 Please wait while we load your content...
Please wait while we load your content...