Solid-phase peptide synthesis on disulfide-linker resin followed by reductive release affords pure thiol-functionalized peptides†
Abstract
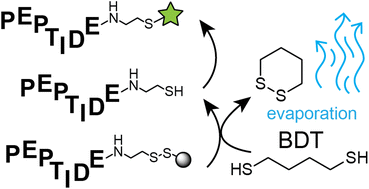
Thiol groups are suitable handles for site-selectively modifying, immobilizing or cyclizing individual peptides or entire peptide libraries. A limiting step in producing the thiol-functionalized peptides is the chromatographic purification, which is particularly laborious and costly if many peptides or even large libraries are to be produced. Herein, we present a strategy in which thiol-functionalized peptides are obtained in >90% purity and free of reducing agent, without a single chromatographic purification step. In brief, peptides are synthesized on a solid support linked via a disulfide bridge, the side-chain protecting groups are eliminated and washed away while the peptides remain on resin, and rather pure peptides are released from the solid support by reductive cleavage of the disulfide linker. Application of a volatile reducing agent, 1,4-butanedithiol (BDT), enabled removal of the agent by evaporation. We demonstrate that the approach is suited for the parallel synthesis of many peptides and that peptides containing a second thiol group can directly be cyclized by bis-electrophilic alkylating reagents for producing libraries of cyclic peptides.


 Please wait while we load your content...
Please wait while we load your content...