Cross-dehydrogenative coupling of ethers and amides with tautomerizable quinazolinones and mechanistic studies†
Abstract
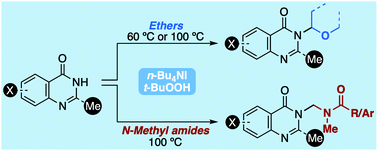
Cross-dehydrogenative coupling reactions have been utilized to alkylate 4(3H)-quinazolinones with ethers and amides, using catalytic n-Bu4NI and t-BuOOH as oxidants. The reactions with amides represent the first examples under such conditions. Studies via inter- and intramolecular competitive experiments with protio and deuterio reactants, as well as radical inhibition experiments, provided mechanistic insight. Also, an understanding of the relative reactivities of ethers was obtained by pairwise competitions with 4(3H)-quinazolinone.


 Please wait while we load your content...
Please wait while we load your content...