Enantioselective synthesis of isoxazole-containing spirooxindole tetrahydroquinolines via squaramide-catalysed cascade reactions†
Abstract
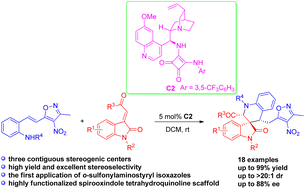
A class of o-sulfonylaminostyryl isoxazole synthons were designed and demonstrated to be useful building blocks in asymmetric cascade aza-Michael/Michael reaction with 3-olefinic oxindoles. This squaramide-catalysed cascade reaction afforded structurally complex isoxazole-containing spirooxindole tetrahydroquinolines bearing three contiguous stereocenters in good to excellent yields (up to 99%) with high diastereoselectivities (up to >20 : 1 dr) and enantioselectivities (up to 88% ee). Moreover, the gram-scale synthesis and synthetic transformations were also demonstrated.


 Please wait while we load your content...
Please wait while we load your content...