TEMPO mediated oxidative annulation of aryl methyl ketones with amines/ammonium acetate for imidazole synthesis†
Abstract
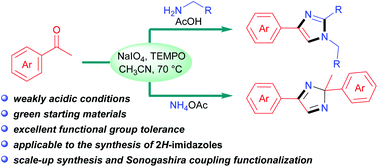
A facile synthesis of 1H-imidazoles by direct oxidative annulation of aryl methyl ketones and primary amines has been developed in the presence of TEMPO under weakly acidic conditions. By replacing amines with ammonium acetate, 2H-imidazole skeletons were achieved for the first time from ketones. Substrates containing various functional groups, such as alkyl, aryl, naphthyl, halogen (F, Cl, Br, I), nitro, trifluoromethyl, sulfonyl ester, furyl, thienyl, and pyridyl groups, were readily transformed into the desired products. The application potential of this method was verified by the scale-up synthesis and Sonogashira coupling functionalization of imidazoles. Mechanistically, the α-TEMPO–enamine adduct may serve as the key reaction intermediate.


 Please wait while we load your content...
Please wait while we load your content...