Substituted pyridines from isoxazoles: scope and mechanism†
Abstract
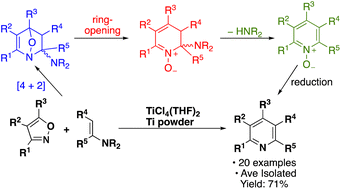
Treatment of isoxazoles with enamines leads to an inverse electron-demand hetero-Diels–Alder reaction that produces substituted pyridines in the presence of TiCl4(THF)2 and titanium powder. The reaction is highly regioselective with only a single isomer of the product observed by GC/MS and tolerant of many common functional groups. The transformation was examined computationally, and it was found that TiCl4 (or a similar Lewis acid) likely acts to catalyze the reaction. After the initial [4 + 2]-cycloaddition, the oxaza-[2.2.1]-bicycle produced likely ring opens before amine loss to give an N-oxide. The pyridine is then obtained after reduction with TiCl4 and titanium powder.


 Please wait while we load your content...
Please wait while we load your content...