Diverse and chemoselective sigmatropic shift rearrangements of multisubstituted N,O-diarylhydroxylamines†
Abstract
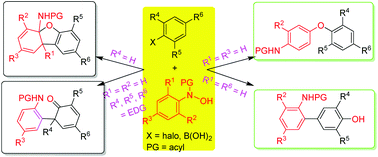
N,O-Diarylhydroxylamines generally favor the [3,3] sigmatropic shift rearrangement. Possible N/O[1,3] sigmatropic shift rearrangements of multisubstituted N,O-diarylhydroxylamines were investigated experimentally with rationally designed substrates, which were generally in situ prepared from suitable nitroaryl halides and N-arylhydroxylamines via aromatic nucleophilic substitution. The results indicate that both N- and O-(2,4,6-trimethylphenyl)hydroxylamines still favor the [3,3] sigmatropic shift followed by tautomerization rather than N[1,3] and O[1,3] sigmatropic shifts and the rearranged products of N-(2,4,6-trimethylphenyl)hydroxylamines further undergo an intramolecular nucleophilic addition to afford dibenzo[b,d]furan-4a(9bH)-amine derivatives, while N-(4-mono- and 3,5-disubstituted phenyl)-O-(2,4,6-trinitrophenyl)hydroxylamines favorably first undergo the O[1,3] sigmatropic shift followed by tandem Smiles rearrangement and amide/ester exchange reactions, generating 2-arylaminoaryl benzoate derivatives. N-Phenyl-O-(2,4,6-trinitrophenyl)hydroxylamines undergo tandem double O[1,3] sigmatropic shift rearrangement to produce formal O[1,5] shift products. However, O-(2,6-dinitrophenyl)-N-(4-substituted phenyl)hydroxylamines undergo tandem O[1,3] and double [3,3] sigmatropic shift rearrangements to give formal 3,5-shift products. The proposed mechanism is rationalized by density functional theory (DFT) calculations. The current investigation provides not only a comprehensive understanding of the chemoselective sigmatropic shift rearrangements of N,O-diarylhydroxylamines, but also some novel synthetic strategies for dibenzo[b,d]furanamines, diarylamines, diaryl ethers, 2′-amino-[1,1′-biphenyl]-2(1H)-one, and 2′-amino-[1,1′-biaryl]-4-ol derivatives.


 Please wait while we load your content...
Please wait while we load your content...