Total synthesis of resolvin D3†
Abstract
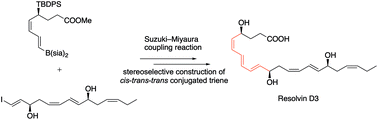
Resolvin D3 was synthesized by the Suzuki–Miyaura cross-coupling reaction of C1–C8 borane with C9–C22 iodoolefin as the key reaction. The latter intermediate was obtained by the sequential Wittig reactions of C9–C13 phosphonium salt with C14–C19 aldehyde and then C9–C19 aldehyde with propyltriphenylphosphonium bromide. The stereogenic centers at C4, C11, and C17 were constructed by the ruthenium-catalyzed asymmetric transfer hydrogenation with high stereoselectivity.


 Please wait while we load your content...
Please wait while we load your content...