Synthesis and Optical Properties of Excited-State Intramolecular Proton Transfer (ESIPT) Emitters with Sulfobetaine Fragments†
Abstract
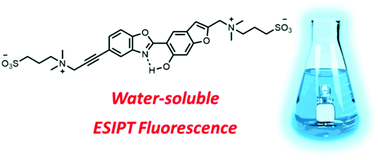
This article describes the synthetic efforts towards the solubilization of organic fluorescent emitters based on a 2-(2′-hydroxybenzofuranyl)benzazole (HBBX) scaffold in aqueous media under physiological conditions (PBS, pH 7.4). These dyes are well-known to display the excited-state intramolecular proton transfer (ESIPT) process which leads to a Stokes-shifted fluorescence with enhanced photostability and strong environment dependent features. Organic dyes are hydrophobic by nature and their vectorization into aqueous media usually necessitates amphiphilic polymers. In this study, we show that the incorporation of one or two sulfobetaine fragments, a highly biocompatible zwitterionic unit leads to the vectorization in buffer solution at pH 7.4 while keeping a reasonable ESIPT fluorescence emission. The photophysical properties of all dyes were studied in multiple solvents and showed that, depending on structure and environment, different excited-state species are observed: normal or tautomeric species, as well as a competitive anionic fluorescent derivative. This study shows that it is not only possible to solubilize fluorescent ESIPT dyes in water using sulfobetaine(s) but also that the optical properties can be finely tuned depending on small structural inputs.


 Please wait while we load your content...
Please wait while we load your content...