Construction of carbazole-based unnatural amino acid scaffolds via Pd(ii)-catalyzed C(sp3)–H functionalization†
Abstract
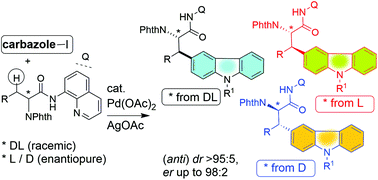
We report the synthesis of carbazole-based unnatural α-amino acid and non-α-amino acid derivatives via a Pd(II)-catalyzed bidentate directing group 8-aminoquinoline-aided β-C(sp3)–H activation/functionalization method. Various N-phthaloyl, DL-, L- and D-carboxamides derived from their corresponding α-amino acids, non-α-amino acids and aliphatic carboxamides were subjected to the β-C(sp3)–H functionalization with 3-iodocarbazoles in the presence of a Pd(II) catalyst to afford the corresponding carbazole moiety installed unnatural amino acid derivatives and aliphatic carboxamides. Carbazole motif-containing racemic (DL) and enantiopure (L and D) amino acid derivatives including phenylalanine, norvaline, leucine, norleucine and 2-aminooctanoic acid with anti-stereochemistry and various non-α-amino acid derivatives including GABA have been synthesized. Removal of the 8-aminoquinoline directing group, deprotection of the phthalimide moiety and the preparation of carbazole amino acid derivatives containing free amino- and carboxylate groups are shown. The carbazole motif is prevalent in alkaloids and biologically active molecules and functional materials. Thus, this work on the synthesis of carbazole-based unnatural amino acid derivatives would enrich the libraries of unnatural amino acid derivatives and carbazoles.


 Please wait while we load your content...
Please wait while we load your content...