Cytotoxic analogues of marine diterpenoid plumisclerin A by shifting the lipophilic branch on the characteristic tricyclic core†
Abstract
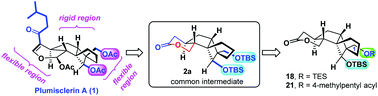
Plumisclerin A is one of the most complex cytotoxic xenicane diterpenes from marine sources, featuring a unique congested and rigid tricyclo[4.3.1.01,5]decane core and a lipophilic acyl chain. This work explored a number of new analogues of plumisclerin A through modifying the characteristic tricyclo[4.3.1.01,5]decane core with lipophilic chains starting from a common lactone intermediate. Bioactivity examination of all the synthetic analogues shows that new analogues 2a, 18 and 21 exhibited comparable inhibitory potencies to that of the natural product against the proliferation of cancer cells. Structural comparison of these bioactive natural and unnatural compounds reveals that the location of lipophilic substituent(s) on the tricyclo[4.3.1.01,5]decane core is spatially flexible, and this work thus offers a new channel to diverse bioactive analogues of plumisclerin A.


 Please wait while we load your content...
Please wait while we load your content...