Synthesis of imidazolocoumarins by the amide-directed oxidative cyclisation of enol-Ugi derivatives†
Abstract
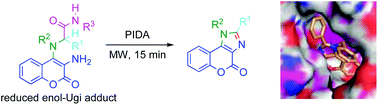
The oxidative C(sp3)–H intramolecular imination of hydroxycoumarin enol-Ugi adduct derivatives affords selectively diversely substituted imidazolocoumarins in one pot. The amide group derived from the enol-Ugi isocyanide component directs the functionalisation of the adjacent C(sp3)–H and then is lost as an isocyanate molecule in an unprecedented transformation. This strategy was applied for the synthesis of potential modulators of innate immune system receptor TLR7, which showed high binding affinities in the molecular docking studies.


 Please wait while we load your content...
Please wait while we load your content...