Synthesis of 3-substituted 2-oxindoles from secondary α-bromo-propionanilides via palladium-catalyzed intramolecular cyclization†
Abstract
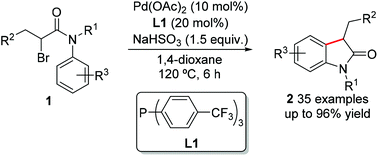
In contrast to aromatic halides, coupling reactions involving oxidative addition of alkyl halides, especially secondary or tertiary halides, to transition metals tend to be more challenging. Herein a palladium-catalyzed intramolecular cyclization of α-bromo-propionanilides has been developed, delivering a series of 3-substituted 2-oxindoles in high yields. The method features easy to prepare starting materials, broad substrate scope and excellent functional group tolerance. A detailed mechanistic investigation has been performed.


 Please wait while we load your content...
Please wait while we load your content...