Multicomponent synthesis of fully substituted thiazoles using glycine-based dithiocarbamates, acetic anhydride and nitroalkenes†
Abstract
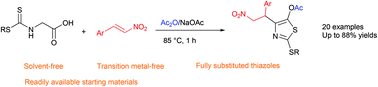
Reaction of glycine-based dithiocarbamates with nitroalkenes in the presence of acetic anhydride was utilized for the synthesis of fully substituted 2-(alkylsulfanyl)-4-(nitroalkyl)-5-acyloxy-1,3-thiazoles. The reaction proceeds via the in situ formation of thiazol-5(4H)-one from glycine-based dithiocarbamates, followed by the Michael addition of this intermediate to nitroalkenes, aromatization, and esterification reaction cascade. This new one-pot three-component reaction afforded a diverse library of fully substituted thiazoles in high to excellent yields under solvent-free conditions.


 Please wait while we load your content...
Please wait while we load your content...