1,2,3-Triazine formation mechanism of the fairy chemical 2-azahypoxanthine in the fairy ring-forming fungus Lepista sordida†
Abstract
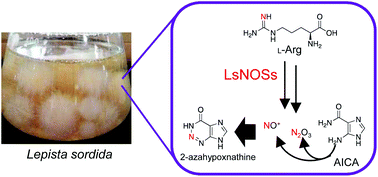
2-Azahypoxanthine (AHX) was first isolated from the culture broth of the fungus Lepista sordida as a fairy ring-inducing compound. It has since been found that a large number of plants and mushrooms produce AHX endogenously and that AHX has beneficial effects on plant growth. The AHX molecule has an unusual, nitrogen-rich 1,2,3-triazine moiety of unknown biosynthetic origin. Here, we establish the biosynthetic pathway for AHX formation in L. sordida. Our results reveal that the key nitrogen sources that are responsible for the 1,2,3-triazine formation are reactive nitrogen species (RNS), which are derived from nitric oxide (NO) produced by NO synthase (NOS). Furthermore, RNS are also involved in the biochemical conversion of 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranosyl 5′-monophosphate (AICAR) to AHX-ribotide (AHXR), suggesting that a novel biosynthetic route that produces AHX exists in the fungus. These findings demonstrate a physiological role for NOS in AHX biosynthesis as well as in biosynthesis of other natural products containing a nitrogen-nitrogen bond.


 Please wait while we load your content...
Please wait while we load your content...