Synthesis of trifluoromethylated aza-BODIPYs as fluorescence-19F MRI dual imaging and photodynamic agents†
Abstract
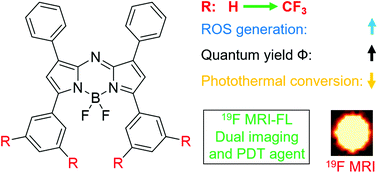
Dual-imaging agents with highly sensitive fluorescence (FL) imaging and highly selective fluorine-19 magnetic resonance imaging (19F MRI) are valuable for biomedical research. At the same time, photosensitizers with a high reactive oxygen species (ROS) generating capability are crucial for photodynamic therapy (PDT) of cancer. Herein, a series of tetra-trifluoromethylated aza-boron dipyrromethenes (aza-BODIPYs) were conveniently synthesized from readily available building blocks and their physicochemical properties, including ultraviolet-visible (UV-Vis) absorption, FL emission, photothermal efficacy, ROS generating efficacy, and 19F MRI sensitivity, were systematically investigated. An aza-BODIPY with 12 symmetrical fluorines was identified as a potent FL-19F MRI dual-imaging traceable photodynamic agent. It was found that the selective introduction of trifluoromethyl (CF3) groups into aza-BODIPYs may considerably improve their UV absorption, FL emission, photothermal efficacy, and ROS generating properties, which lays the foundation for the rational design of trifluoromethylated aza-BODIPYs in biomedical applications.


 Please wait while we load your content...
Please wait while we load your content...