One-pot synthesis of dihydroquinolones by sequential reactions of o-aminobenzyl alcohol derivatives with Meldrum's acids†
Abstract
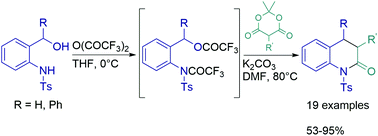
The functionalized 3,4-dihydroquinolin-2-one nucleus has been assembled in good to high yields through the sequential reaction of readily available N-Ts-o-aminobenzyl alcohols with 5-substituted Meldrum's acid derivatives under mild basic conditions. Highly diastereoselective synthesis of 3-substituted-4-phenyl-1-tosyl-3,4-dihydroquinolin-2(1H)-ones was accomplished from N-(2-(hydroxy(phenyl)methyl)phenyl)-4-methylbenzenesulfonamide under the same reaction conditions. Regarding the reaction mechanism, we hypothesized that the formation of dihydroquinolones proceeds through the in situ generation of aza-o-QMs followed by conjugate addition of enolate/cyclization/elimination of acetone and CO2.


 Please wait while we load your content...
Please wait while we load your content...