Synthesis of 1,3-diselenyl-dihydroisobenzofurans via electrochemical radical selenylation with substituted o-divinylbenzenes and diselenides†
Abstract
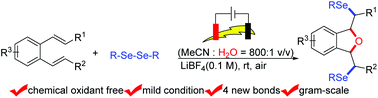
An efficient electrochemical method for the direct synthesis of complicated 1,3-diselenyl-dihydroisobenzofurans was developed under external oxidant free conditions at room temperature from substituted o-divinylbenzenes and diselenides. A radical mechanism is proposed for this novel and practical transformation.

 Please wait while we load your content...
Please wait while we load your content...