Convergent biomimetic semisynthesis of disesquiterpenoid rumphellolide J†
Abstract
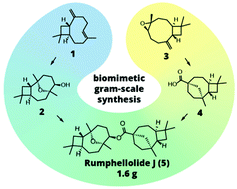
The convergent biomimetic gram-scale synthesis of disesquiterpenoid ester rumphellolide J is described. 4β,8β-Epoxycaryophyllan-5-ol was prepared in 67% yield (1.4 g) from naturally ambudant (−)-β-caryophyllene. (+)-Rumphellaoic acid A was obtained in 46% yield (2.2 g) from (−)-caryophyllene oxide. The synthesised (+)-rumphellaoic acid had an opposite specific rotation compared to that of (−)-rumphellaoic acid A isolated from nature, indicating possible occurrence of (+)-β-caryophyllene in Rumphella antipathies and Psidium guajava. Esterification of (+)-rumphellaoic acid A via acyl fluoride and alkoxide of 4β,8β-epoxycaryophyllan-5-ol gave rumphellolide J in 70% yield (1.65 g). The same structure for the synthesized product and natural isolate was proven despite the opposite specific rotation value of the intermediate acid. The short access to the terpenoids provides a material for further investigations of biological activities and valuable reference standards for the analysis of the chemical composition of various natural sources.

 Please wait while we load your content...
Please wait while we load your content...