Total synthesis of (±) commiphoranes C–D and their epimers†
Abstract
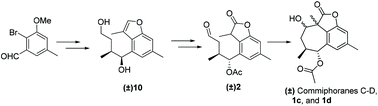
(±) Commiphorane C, (±) commiphorane D, and their two isomers were synthesized through a linear synthesis strategy in 14 steps. Key features of the strategy include the construction of the relative configurations of C-5 and C-6 via aldehyde crotylation followed by the Mitsunobu reaction and ring A via an intramolecular Aldol reaction. The biological evaluation revealed that (±) commiphorane C and (±) isomer-1 significantly attenuated the overproduction of fibronectin, collagen I, and α-SMA in TGF-β1-induced rat renal proximal tubular cells. Intermediate (±) 11 significantly decrease the overexpression of collagen I. Cytotoxicity studies showed that 1a–1d and (±) 11 were not toxic to NRK-52E cells.


 Please wait while we load your content...
Please wait while we load your content...