Metal-free oxidative formal C(sp2)–H arylation of cyclopentene-1,3-diones with β-naphthols†
Abstract
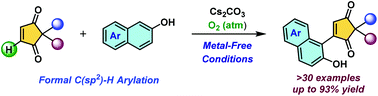
An efficient, mild, and transition-metal free formal C(sp2)–H arylation of prochiral 2,2-disubstituted cyclopentene-1,3-diones is reported. This oxidative arylation with β-naphthols proceeds via base-mediated C-Michael addition followed by aerial oxygen insertion and a subsequent α-hydroperoxy elimination sequence. This operationally simple and environmentally benign transformation is highly scalable and does not require any pre-functionalization. Moreover, this reaction affords excellent yields of α-substituted β-naphthols bearing all-carbon quaternary centers.


 Please wait while we load your content...
Please wait while we load your content...