Computational insight into the mechanism and stereoselectivity of cycloaddition between donor–acceptor spirocyclopropane and aldehyde catalyzed by Brønsted acid TsOH†
Abstract
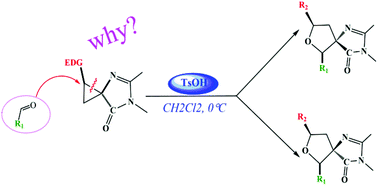
The mechanism and diastereoselectivity of the cycloaddition reaction between D–A spirocyclopropane and aldehydes, catalyzed by para-toluenesulfonic acid (TsOH) in dichloromethane to produce 2,5-disubstituted tetrahydrofuran-type lignans, have been investigated by density functional theory (DFT) at the M06-2X/6-311+G(d,p)//B3LYP-D3/6-31G(d,p) level combined with the solvation SMD model. Our calculations show that the entire reaction process includes three stages: the activation of the D–A cyclopropane by Brønsted acid, TsOH, the nucleophilic attack of the aldehyde on the spirocyclopropane, and the formation of the final product, 2,5-disubstituted tetrahydrofuran. It was concluded from the conceptual density functional theory (CDFT) reactivity index analysis that aldehydes with electron-rich substituents are more nucleophilic and more favorable for the reaction to proceed. Furthermore, based on the analyses of energetics as well as the noncovalent interaction (NCI) and reduced density gradient (RDG) in the key transition states, the origin of stereoselectivity was revealed to be determined thermodynamically rather than kinetically. The present work explains the experimental phenomenon well, and provides useful theoretical information for the future design of similar reactions.


 Please wait while we load your content...
Please wait while we load your content...