Stereoselective synthesis of tri-substituted tetrahydrothiophenes and their in silico binding against mycobacterial protein tyrosine phosphatase B†
Abstract
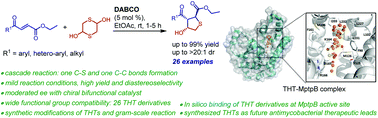
A facile approach to tri-substituted tetrahydrothiophenes via thia-Michael/aldol has been developed. The cascade reaction was carried out in the presence of 5 mol% of DABCO in ethyl acetate to afford diversely functionalized tetrahydrothiophenes (THTs) with excellent diastereoselectivity. The present methodology has broad substrate tolerance. Gram-scale reaction proceeds with equal efficiency. Functional group transformations further highlight the synthetic potential of the THTs. An asymmetric version of the cascade reaction has also been investigated and a maximum of 72% ee was observed with cinchonidine derived squaramide. Moreover, in silico based molecular docking followed by deep learning based affinity prediction and molecular dynamics simulation analysis indicate the synthesized THT derivatives can act as potent competitive inhibitors of MptpB at low micromolar to nanomolar concentrations. In silico ADME analysis further suggests the plausibility of these compounds to act as future anti-mycobacterial therapeutic leads.


 Please wait while we load your content...
Please wait while we load your content...