Convergent synthesis of (+)-carambolaflavone A, an antidiabetic agent using a bismuth triflate-catalyzed C-aryl glycosylation†
Abstract
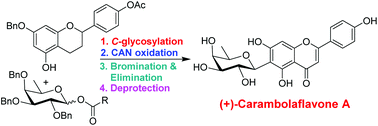
We describe a convergent total synthesis of carambolaflavone A, a natural flavonoid C-aryl glycoside with significant antihyperglycemic properties. The synthesis features a bismuth triflate-catalyzed stereoselective C-aryl glycosylation of a flavan derivative and an appropriately protected D-fucose derivative as the key step. Inexpensive and non-toxic bismuth triflate provided the best results among various other Lewis acids screened for this C-aryl glycosylation. The method can be utilized for the synthesis of other bioactive C-glycosyl flavonoids. The glycosylation partners were synthesized from commercially available (±)-naringenin and D-(+)-galactose, respectively. An oxidative bromination and elimination reaction sequence was utilized to construct the flavone. The natural product is obtained in 13 steps (longest linear sequence) from D-(+)-galactose.

 Please wait while we load your content...
Please wait while we load your content...