Recent progress in asymmetric synergistic catalysis – the judicious combination of selected chiral aminocatalysts with achiral metal catalysts
Abstract
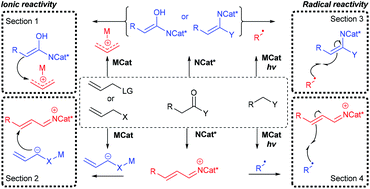
In this review we survey recent synergistic applications of a chiral organocatalyst with an achiral metal to perform stereoselective transformations of synthetic utility (since 2016). The transformations are classified by the modes of reactivity deployed, focussing on organocatalytic activation of carbonyl substrates as chiral nucleophiles via the α-position (e.g., as enamines) and as chiral electrophiles via the β-position (e.g., as iminium ions) combined with complementary activation of their reaction partners by an achiral metal co-catalyst (e.g., Pd or Cu-based). Corresponding radical reactions are also presented in which photocatalysis mediated by achiral metal complexes replaces the metal co-catalyst. Certain privileged structures are revealed and opportunities to develop this exciting field are highlighted.

 Please wait while we load your content...
Please wait while we load your content...