Synthesis of phenoxathiins using an iron-catalysed C–H thioarylation†
Abstract
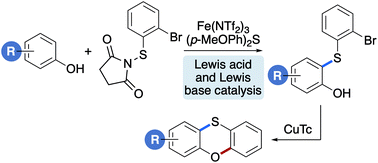
Phenoxathiins are an important class of sulfur-containing heterocycle, found as the core component in numerous pharmaceutically active agents and materials. Despite this importance, there are relatively few methods for the synthesis of these heterocycles that avoid complex starting materials, harsh conditions or precious transition metals. We report a two-step synthesis of phenoxathiins from phenols using iron and copper-mediated reactions. The first step involves the accelerated ortho-thioarylation of phenols using N-(2-bromophenylthio)succinimide, catalysed by the Lewis acid, iron(III) triflimide and the Lewis base, bis(4-methoxyphenyl)sulfane. In the second step, the thioarylated products were converted to a series of phenoxathiins using a copper-mediated, Ullmann-type, C–O bond forming cyclisation reaction. The synthetic utility of this two-step approach for the preparation of biologically relevant phenoxathiins was demonstrated using natural product-based phenols.


 Please wait while we load your content...
Please wait while we load your content...