Efficient synthesis of an apremilast precursor and chiral β-hydroxy sulfones via ketoreductase-catalyzed asymmetric reduction†
Abstract
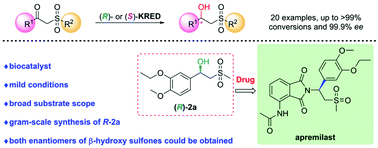
Ketoreductase (KRED)-catalyzed asymmetric reduction of prochiral ketones is an attractive method to synthesize chiral alcohols. Herein, two KREDs LfSDR1-V186A/E141I and CgKR1-F92I with complementary stereopreference were identified towards reduction of apremilast prochiral ketone intermediate 1a. LfSDR1-V186A/E141I exhibited >99% conversion and 99.2% ee yielding an apremilast chiral alcohol intermediate ((R)-2a) at 50 g L−1 substrate loading. Furthermore, we investigated the substrate scope of β-keto sulfones by using LfSDR1-V186A/E141I and CgKR1-F92I to produce both enantiomers of the corresponding β-hydroxy sulfones, with good-to-excellent conversion (up to >99%) and enantioselectivity (up to 99.9% ee) being obtained in most cases. Finally, the gram-scale synthesis of (R)-2a was performed by employing the crude enzyme of LfSDR1-V186A/E141I and BsGDH to afford the desired enantiomer with >99% conversion, 85.9% isolated yield and 99.2% ee. This study presents a biocatalytic strategy to synthesize chiral β-hydroxy sulfones.


 Please wait while we load your content...
Please wait while we load your content...