Controlled reduction of activated primary and secondary amides into aldehydes with diisobutylaluminum hydride†
Abstract
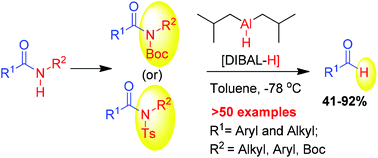
A practical method is disclosed for the reduction of activated primary and secondary amides into aldehydes using diisobutylaluminum hydride (DIBAL-H) in toluene. A wide range of aryl and alkyl N-Boc, N,N-diBoc and N-tosyl amides were converted into the corresponding aldehydes in good to excellent yields. Reduction susceptible functional groups such as nitro, cyano, alkene and alkyne groups were found to be stable. Broad substrate scope, functional group compatibility and quick conversions are the salient features of this methodology.


 Please wait while we load your content...
Please wait while we load your content...