A case study of the MAC (masked acyl cyanide) oxyhomologation of N,N-dibenzyl-l-phenylalaninal with anti diastereoselectivity: preparation of (2S,3S)-allophenylnorstatin esters†
Abstract
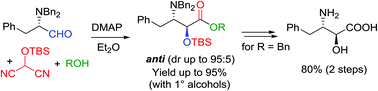
The three-component reaction between a protected α-amino aldehyde, an alcohol and an α-silyloxymalononitrile provides an expedient access to protected α-hydroxy-β-amino acid derivatives. The prototypical process, performed on N-Cbz-phenylalaninal, is known to proceed with syn diastereoselectivity. The present study demonstrates that the diastereoselectivity of the reaction can be inverted, using the rationale of a Felkin-Anh interaction model. Reactions performed on N,N-dibenzyl-L-phenylalaninal proceed with a high anti diastereoselectivity, providing a panel of synthetically useful ester derivatives of (2S,3S)-allophenylnorstatin. The procedure is exploited to accomplish one of the most efficient syntheses of the title compound to date, in 3 steps (66% yield) from N,N-dibenzyl-L-phenylalaninal.


 Please wait while we load your content...
Please wait while we load your content...