Transaminases as suitable catalysts for the synthesis of enantiopure β,β-difluoroamines†
Abstract
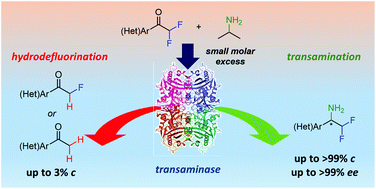
Transaminases have shown the ability to catalyze the amination of a series of aliphatic and (hetero)aromatic α,α-difluorinated ketones with high stereoselectivity, thus providing the corresponding β,β-difluoroamines in high isolated yields (55–82%) and excellent enantiomeric excess (>99%). It was also observed that these activated substrates could be quantitatively transformed by employing a small molar excess of the amine donor since this amination process was thermodynamically favored. Selected transformations could be scaled up to 500 mg, showing the robustness of this methodology.


 Please wait while we load your content...
Please wait while we load your content...