N-Alkylation of organonitrogen compounds catalyzed by methylene-linked bis-NHC half-sandwich ruthenium complexes†
Abstract
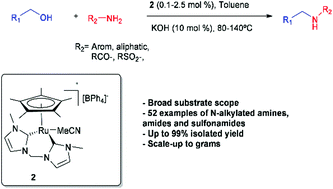
An efficient ruthenium-catalyzed N-alkylation of amines, amides and sulfonamides has been developed employing novel pentamethylcyclopentadienylruthenium(II) complexes bearing the methylene linked bis(NHC) ligand bis(3-methylimidazol-2-ylidene)methane. The acetonitrile complex 2 has proven particularly effective with a broad range of substrates with low catalyst loading (0.1–2.5 mol%) and high functional group tolerance under mild conditions. A total of 52 N-alkylated organonitrogen compounds including biologically relevant scaffolds were synthesized from (hetero)aromatic and aliphatic amines, amides and sulfonamides using alcohols or diols as alkylating agents in up to 99% isolated yield, even on gram-scale reactions. In the case of sulfonamides, it is the first example of N-alkylation employing a transition-metal complex bearing NHC ligands.
- This article is part of the themed collection: Synthetic methodology in OBC


 Please wait while we load your content...
Please wait while we load your content...