Self-standing Fe3O4 decorated paper electrode as a binder-free trifunctional electrode for electrochemical ammonia synthesis and Zn–O2 batteries†
Abstract
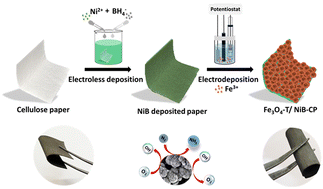
The conversion of the abundant biodegradable material into electroactive electrode material can be a good resource for sustainable energy conversion and storage applications. Herein, we present a simple, cost-effective and green approach for the fabrication of a flexible cellulose paper electrode using an electroless-electrodeposition method. The one-step electroless deposition route is followed to induce conductivity into a non-conductive cellulose paper substrate without using any expensive activators or sensitisers. The Fe3O4 is then electro-deposited as an active catalyst over the conductive paper substrate for use in electrochemical activities. The as-fabricated paper electrode shows promising activity and stability during the dinitrogen reduction reaction (NRR) as well as oxygen bifunctional electrocatalysis. A faradaic efficiency of 4.32% with a yield rate of 245 μg h−1 mgcat−1 at −0.1 V is achieved for NRR whereas a very small overpotential of 180 mV is required to reach 10 mA cm−2 during OER, and the ORR reaction starts at the onset potential of 0.86 V. The practical applicability of the paper electrode is validated by assembling a Zn–O2 battery showing a peak power density of 81 mW cm−2 and a stability up to 35 h during charge–discharge cycles, which can power the NRR to produce NH3 under full cell conditions.


 Please wait while we load your content...
Please wait while we load your content...