Molybdenum-iron–cobalt oxyhydroxide with rich oxygen vacancies for the oxygen evolution reaction†
Abstract
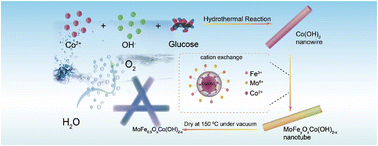
The sluggish kinetics of the oxygen evolution reaction (OER) restrains the development of water splitting technologies and the efficiency of producing sustainable resources. To this end, the introduction of iron and molybdenum in catalytic systems has been employed as a crucial strategy for the enhancement of catalytic activity toward the oxygen evolution reaction (OER), but the relationship between catalyst components and catalytic performance is still evasive. In this study, by doping iron and molybdenum into cobalt hydroxide via a cation-exchange method, rich oxygen vacancies and active metal centers are introduced to the trimetallic oxyhydroxide, endowing the catalyst with a low overpotential of 223 mV at 10 mA cm−2, a low Tafel slope of 43.6 mV dec−1, and a long stable operation time (>50 h) in alkaline media, comparable to the current best OER catalyst. Moreover, it is demonstrated that the doping of iron favors the generation of oxygen vacancies. It is also found in this work that using a certain amount (5 mg) of iron dopant can alter the electronic structure of the catalyst by tuning the electronic density around the metal ions, thus optimizing the binding energy of intermediates. The present work unveils the doping effect of iron and molybdenum on the construction of trimetallic oxyhydroxide catalysts, and sheds light on the relationship between the catalyst components and catalytic performance of the OER.


 Please wait while we load your content...
Please wait while we load your content...